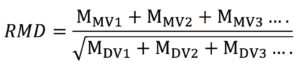courtesy of Minerals Technologies Inc.
Geosynthetic clay liners (GCL) and polymer-modified geosynthetic clay liners (PMG) are gaining favor for use in industrial waste disposal and ore-processing applications (Athanassopoulos et al. 2015, Donovan et al. 2016a, Donovan et al. 2016b). The GCL or PMG can be a component of a composite liner system that will allow for increased performance (relative to compacted clay) as well as a more environmentally responsible and economical design. The performance advantages afforded by GCL will minimize the risk of leakage into the environment. Additionally, GCL and PMG can provide manufacturing quality assurance (MQA) and ease of installation (Figure 1).
Traditional sodium bentonite GCL function best in conditions that promote the swelling and sealing power of the hydrated montmorillonite platelets of the clay. However, applications that involve high ionic strength or high pH conditions can preclude the use of traditional sodium bentonite GCL due to the reduced swelling potential of the bentonite. Trona mining, bauxite mining and cement kiln dust (CKD) storage are applications that have both high dissolved salt concentrations and high alkalinity. Trona (also referred to as sodium sesquicarbonate) is mined to produce soda ash that is used in commercial applications/products such as baking soda, glass production and flue gas desulfurization agents. In trona mining, mining process waste water is piped to surface impoundments or ponds. In a similar manner, red mud waste generated by the chemical leaching process of bauxite for aluminum production is stored behind tailings dams. Red mud must be stored indefinitely due to the hazardous nature of the waste. Recent high-profile red mud storage dam failures have led to improved storage designs using composite liners. CKD is another highly alkaline type of waste. CKD is a waste product that is removed from cement kiln exhaust gas by air pollution control devices. Some of the CKD that is not recycled in the cement manufacturing process is disposed in landfills, waste piles or surface impoundments. When this dust mixes with water, it can be corrosive and promote the mobility of heavy metals into the soils surrounding a storage facility. Other risks associated with the leaching of CKD is the presence of heavy metals including arsenic, cadmium, chromium, thallium and lead, as well as the presence of dioxin in the dust.
New clay liner technologies have been developed to extend the use of a manufactured liner into such aggressive applications. Silica-modified bentonites as well as polymer-modified bentonite technologies have been successful in these applications. A particularly interesting aspect of the engineering and design of the liner has been the type of bentonite that is suitable for use. Studies by Benson et al. (2008) and Bouazza and Gates (2014) have found that bentonites with a naturally high concentration of silica can yield lower hydraulic conductivities compared to traditional bentonites that meet performance criteria, such as GRI-GCL3, the standard specification for “Test Methods, Required Properties, and Testing Frequencies of Geosynthetic Clay Liners (GCLs),” developed by the Geosynthetic Research Institute (Geosynthetic Institute 2016). Benson et al. (2008) and Bouazza and Gates (2014) demonstrated that the accessory minerals associated with the specific bentonite source can result in varying hydraulic performance. For this study, bentonites sourced from the U.S., China, India and Turkey were mixed with a proprietary polymer treated package, and compared for the chemical compatibility with various bauxite, trona and CKD leachates. Also, we sought to understand the influence of mass per unit area on the performance of systems, which ranged from 0.76 to 1.09 pounds/square foot (3.7–5.3 kg/m2).
ICP testing
Inductively coupled plasma (ICP) testing was performed on a Thermo Scientific iCAP 7000 Series ICP spectrometer unit equipped with a radial argon torch. The TEVA 1.6.5 software was used to collect the data. Prior to testing, the bauxite leachates were diluted 1:100 with de-ionized water. The trona leachates were diluted 1:1000 with deionized water. The ICP testing was calibrated with standard electrolyte solution: 5, 50, 100 and 200 ppm prior to analysis. The leachate samples were analyzed for calcium, aluminum, manganese, magnesium, iron, zinc, potassium sulfur, phosphorus, copper and silicon.
Hydraulic conductivity
Hydraulic conductivity tests on GCL specimens were conducted in a flexible-wall permeameter using a falling headwater/constant tail water method, as described in ASTM D6766-12 (ASTM 2012). The GCL were hydrated with permeant liquid in the permeameter for 48 hours at an effective confining stress of 1.45 pounds/square inch (10 kPa). After prehydration, the effective confining stress was increased to 2.9 pounds/square inch (20 kPa), and the hydraulic gradient was set at approximately 150. Influent for the specimen was introduced using a burette. The permeate was collected in sealed individual vials. Permeability testing was conducted for varying lengths of time depending on the hydraulic properties of the sample.
Leachate characterization
Determination of electrical conductivity (EC) was performed using a Mettler Toledo SevenGo Pro conductivity meter. The EC was expressed as microsiemens per centimeter (µS/cm). The pH of the leachates and permeates were measured using an Oakton Ion 700 pH meter equipped with an Oakton Acorn model 35811-98 probe. Chloride contents were estimated by QuanTab® Test Strips. The sulfate content was estimated by the sulfur response detected by ICP.
Ionic strength (I) is calculated using Equation 1:
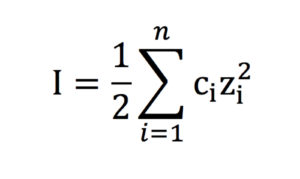
The ionic strength, I, of a solution is a function of the concentration of all ions present, where ci is the molar concentration of ion i (mol/L), zi is the charge number of that ion and the sum is calculated from all ions in the solution.
Ratio of monovalent to divalent ions (RMD) is calculated using Equation 2:
Relative abundance of monovalent and multivalent cations was characterized by the RMD of each test solution. The RMD is defined as the ratio of the total molarity of monovalent cations to the square root of the total molarity of multivalent cations at a given ionic strength. ICP data was used to estimate the RMD of the leachate.
 Polymer-modified geosynthetic clay liner (PMG)
Polymer-modified geosynthetic clay liner (PMG)
PMG-4 is the fourth of a series of PMG technologies developed by the authors. Samples of PMG-4 were produced by using a commercial needlepunching loom using a proprietary manufacturing process. The bentonites were sourced from several countries, including the U.S., Turkey, India and China. With the exception of the U.S. bentonite, the clays used were soda-ash-activated calcium bentonites. The bentonites were of similar grain-size distribution. The clays were granular clays with a maximum 10% retained on 18 mesh (0.033 inch [850 µm]) and maximum 15% passing 200 mesh (0.003 inch [75 µm]). The average inherent moisture content of the bentonite used was 10–12%. The polymer treat package used is a proprietary material developed by the authors. The PMG were produced with a range of mass per unit area (dry content) from 0.76 to 1.09 pounds/square foot (3.7–5.3 kg/m2). The inherent water content of the bentonite was included in the mass target and blending calculations. The bentonite and polymer were combined using a proprietary process and incorporated into polypropylene geotextile fabrics typical for GCL.
 Leachates
Leachates
The trona leachate T1 was sent from a commercial trona located in Wyoming. Synthetic CKD leachates were also prepared in our lab. CKD1 was prepared by dissolving 1.8 ounces (50 g) of sodium chloride and 0.5 ounce (14.1 g) of potassium hydroxide in deionized water up to a total volume of 0.26 gallon (1 L). CKD2 was prepared by dissolving 0.16 ounce (4.5 g) potassium sulfate, 1.33 ounces (37.72 g) potassium chloride, 0.21 ounce (5.97 g) sodium chloride, 0.03 ounce (0.82 g) magnesium chloride, 0.15 ounce (4.32 g) calcium chloride and 0.2 ounce (5.6 g) potassium hydroxide in deionized water up to a total volume of 0.26 gallon (1 L). Synthetic bauxite leachates were also prepared in our lab. Bauxite liquor B1 was prepared by dissolving 1.31 ounces (37.14 g) sodium hydroxide, 1.62 ounces (45.88 g) aluminum chloride hexahydrate, 0.01 ounce (0.27 g) potassium chloride and 0.23 (6.6 g) sodium sulfate in deionized water up to a total volume of 0.26 gallon (1 L). A sodium chloride leachate (NaCl1) was prepared by dissolving 1.8 ounces (50 g) sodium chloride in deionized water up to a total volume of 0.26 gallon (1 L). A 50% sodium hydroxide solution was also prepared in deionized water.
 Results and discussion
Results and discussion
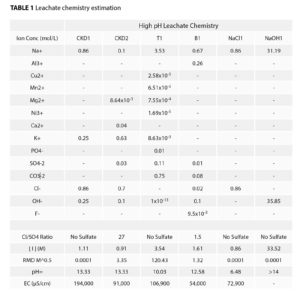
It is well known that traditional GCL hydraulic conductivity is influenced by several factors, including ionic strength and RMD, in addition to the design parameters of the GCL, such as bentonite properties and mass per unit area. Ideal PMG systems would be those that are tolerant to a wide range of leachate chemistries. The swelling pressures generated by hydrated bentonite along with the polymer/solvent and polymer/clay interactions all factor into the performance of the system. As indicated by a large body of work investigating the interactions of ions and macromolecules in solution (known as Hofmeister effects), we intend to track both the influence of both anions and cations on hydraulic performance. For this work, estimations of the ionic strength and RMD are shown in Table 1 for each leachate. The ionic strengths ranged from 0.86 to 4.16 mol/L. The RMD values are elevated due to the high concentrations of sodium ions typical for these applications.
 The solutions have varying anion species, and relative concentrations range from 100% chloride to a mixture of chlorides, sulfates, carbonates, etc. With the exception of the NaCl1 leachate, pH of the systems ranged from 9.7 to 13.3. Elevated pH values are known to have a negative influence on the hydraulic conductivity of traditional bentonite GCL. The higher ionic strength (imparted by the concentration of hydroxide ions) can reduce the electrostatic repulsion of the montmorillonite platelets. At high pH values, the silicate layer of the montmorillonite can be chemically digested, which further reduces the swelling potential of the clay.
The solutions have varying anion species, and relative concentrations range from 100% chloride to a mixture of chlorides, sulfates, carbonates, etc. With the exception of the NaCl1 leachate, pH of the systems ranged from 9.7 to 13.3. Elevated pH values are known to have a negative influence on the hydraulic conductivity of traditional bentonite GCL. The higher ionic strength (imparted by the concentration of hydroxide ions) can reduce the electrostatic repulsion of the montmorillonite platelets. At high pH values, the silicate layer of the montmorillonite can be chemically digested, which further reduces the swelling potential of the clay.
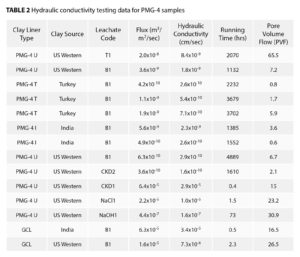
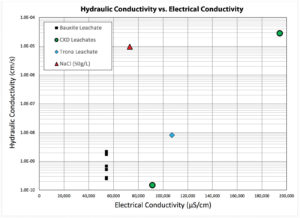
 As noted earlier, PMG-4 is the fourth of a series of PMG technologies developed by the authors. The hydraulic conductivity of the tested GCL is shown in Table 2. The samples were tested for pore volumes of flow and times. Figure 2 depicts the hydraulic conductivity plotted as a function of electrical conductivity. With the exception of the NaCl1 solution, the system exhibited low hydraulic conductivity values (<1 × 10-8 cm/sec) up to 133,000 µS/cm. From this data it appears that solutions with higher chloride ion content are more aggressive. Interestingly, the NaOH1 solution, despite having the most aggressive conditions, exhibited a hydraulic conductivity of 1.6×10-7 cm/sec. This was most likely attributed to the higher viscosity of that particular leachate.
As noted earlier, PMG-4 is the fourth of a series of PMG technologies developed by the authors. The hydraulic conductivity of the tested GCL is shown in Table 2. The samples were tested for pore volumes of flow and times. Figure 2 depicts the hydraulic conductivity plotted as a function of electrical conductivity. With the exception of the NaCl1 solution, the system exhibited low hydraulic conductivity values (<1 × 10-8 cm/sec) up to 133,000 µS/cm. From this data it appears that solutions with higher chloride ion content are more aggressive. Interestingly, the NaOH1 solution, despite having the most aggressive conditions, exhibited a hydraulic conductivity of 1.6×10-7 cm/sec. This was most likely attributed to the higher viscosity of that particular leachate.
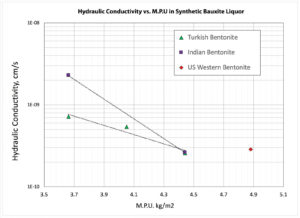
 Hydraulic conductivity as a function of mass per unit area for the PMG-4 system in the synthetic bauxite liquor is plotted in Figure 3. The hydraulic conductivity decreases from ~2 × 10-9 cm/sec at 0.76 pound/square foot (3.7 kg/m2) for the Indian bentonite to ~3 × 10-10 cm/sec at 0.92 pound/square foot (4.5 kg/m2). The Turkish bentonite gave slightly lower k values at the lower loadings. Higher loadings at 1 pound/square foot (4.9 kg/m2) did not appear to yield lower k values.
Hydraulic conductivity as a function of mass per unit area for the PMG-4 system in the synthetic bauxite liquor is plotted in Figure 3. The hydraulic conductivity decreases from ~2 × 10-9 cm/sec at 0.76 pound/square foot (3.7 kg/m2) for the Indian bentonite to ~3 × 10-10 cm/sec at 0.92 pound/square foot (4.5 kg/m2). The Turkish bentonite gave slightly lower k values at the lower loadings. Higher loadings at 1 pound/square foot (4.9 kg/m2) did not appear to yield lower k values.
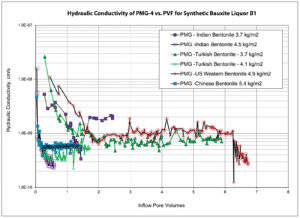
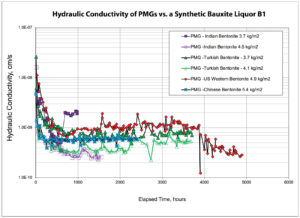
Bauxite leachate chemistries generated by processing methods such as the Bayer process are well documented. Changes to methods used in the industry are resulting in higher dissolved salt concentrations. The leachate chosen for this testing (B1) is considered representative in terms of ionic strength and RMD. Figure 4 shows the hydraulic conductivity of PMG-4 systems made with different regionally sourced bentonite clays for the bauxite leachate (B1) as a function of pore volume flow (PVF). From the data, the hydraulic equilibrium occurs somewhere between 2 PVF and 7 PVF. In regards to the duration required to reach hydraulic equilibrium, the same data is plotted in Figure 5 as a function of time. The experiments showed similar behavior in that they required approximately 890 hours (37 days) to reach hydraulic equilibrium.
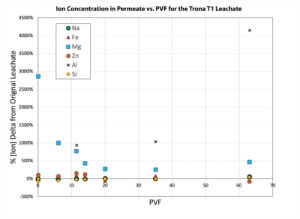
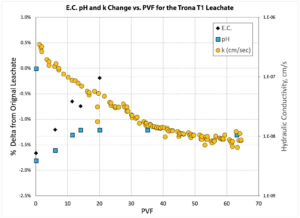 The chemical equilibrium data was tracked for the trona leachate (T1) permeability testing with the PMG-4 U system (Western U.S. Sodium Bentonite). The pH and electrical conductivity values, known as traditional indicators for measuring chemical equilibrium, are plotted in Figure 6 as a function of PVF. Also plotted on the secondary axis is the hydraulic conductivity, which reached equilibrium at approximately 53 PVF. Interestingly, the EC and pH measurements did not show large variances from the original leachate and were lower during most of the experiment by a small amount compared to the original leachate. The point of chemical equilibrium can also be tracked by following the changes in the chemistry of the permeate as a function of PVF. The elemental composition of the permeate was determined using inductively coupled plasma. The ions that were investigated in the permeate were those known to be associated with phyllosilicates such as montmorillonite. These ions were chosen so as to give an indication of the chemical digestion of the bentonite itself. Shown in Figure 7 are the plots of the ion concentration changes relative to the molar concentration of the incoming leachate. Interestingly, the concentration of aluminum increased during the entire experiment. Ions such as magnesium, zinc and iron reached a steady state at approximately 20 PVF. Ions such as sodium and silicon did not fluctuate much during the experiment, which may indicate the precipitation of the silicon as seen in the prior work by Bouazza and Gates (2014).
The chemical equilibrium data was tracked for the trona leachate (T1) permeability testing with the PMG-4 U system (Western U.S. Sodium Bentonite). The pH and electrical conductivity values, known as traditional indicators for measuring chemical equilibrium, are plotted in Figure 6 as a function of PVF. Also plotted on the secondary axis is the hydraulic conductivity, which reached equilibrium at approximately 53 PVF. Interestingly, the EC and pH measurements did not show large variances from the original leachate and were lower during most of the experiment by a small amount compared to the original leachate. The point of chemical equilibrium can also be tracked by following the changes in the chemistry of the permeate as a function of PVF. The elemental composition of the permeate was determined using inductively coupled plasma. The ions that were investigated in the permeate were those known to be associated with phyllosilicates such as montmorillonite. These ions were chosen so as to give an indication of the chemical digestion of the bentonite itself. Shown in Figure 7 are the plots of the ion concentration changes relative to the molar concentration of the incoming leachate. Interestingly, the concentration of aluminum increased during the entire experiment. Ions such as magnesium, zinc and iron reached a steady state at approximately 20 PVF. Ions such as sodium and silicon did not fluctuate much during the experiment, which may indicate the precipitation of the silicon as seen in the prior work by Bouazza and Gates (2014).
 Conclusion
Conclusion
The hydraulic conductivity of a new polymer-modified GCL (PMG-4) was evaluated for high pH leachates. For the PMG-4 system, the maximum electrical conductivity that allowed for low hydraulic conductivities was ~133,000 µS/cm. The regional source of bentonite did not have a major influence on the hydraulic conductivity. Higher mass per unit area results in lower hydraulic conductivity values for the synthetic bauxite leachate. Depending on the leachate tested, the hydraulic equilibrium occurred at different points in the experiments. For bauxite leachate testing, hydraulic equilibrium occurred at 2 pore volumes of flow (PVF). For trona leachate testing, hydraulic equilibrium occurred at 53 PVF. In addition, the ion chemistry changes as the function of PVF was dependent upon the type of ion for the trona leachate. Our work indicates that the ultimate permeability, hydraulic equilibrium and chemical equilibrium is leachate specific. Testing must be conducted to a sufficient pore volume of flow to be confident the system has reached a steady state.
Michael S. Donovan is global R&D director at Minerals Technologies Inc. in Bethlehem, Pa.
Robert Valorio is global technical services director at Minerals Technologies Inc.
Barbara Gebka is senior research associate at Minerals Technologies Inc.
References
ASTM. (2012). “ASTM D6766-12: Standard test method for evaluation of hydraulic properties of geosynthetic clay liners permeated with potentially incompatible aqueous solutions,” D35.04, 04.13, American Society of Testing and Materials, West Conshohocken, Pa.
Athanassopoulos, C., Benson, C., Chen, J., and Donovan, M. (2015). “Hydraulic conductivity of a polymer-modified GCL permeated with high-pH solutions.” Proc., 2015 Geosynthetics Conf., Industrial Fabrics Association International, Roseville, Minn., 181–186.
Benson, C., Wang, X., Gassner, F., and Foo, D. (2008). “Hydraulic conductivity of two geosynthetic clay liners permeated with an aluminum residue leachate.” Proc., GeoAmericas 2008, Industrial Fabrics Association International, Roseville, Minn., 94–101.
Bouazza, A., and Gates, W. (2014). “Overview of performance compatibility issues of GCLs with respect to leachates of extreme chemistry.” Geosynthetics International, 21(2), 151–167.
Donovan, M., Valorio, R., and Gebka, B. (2016a) “Polymer enhanced geosynthetic clay liners for extreme leachate chemistries.” Proc., EuroGeo6 Conf., International Geosynthetics Society, Jupiter, Fla.
Donovan, M., Valorio, R., and Gebka, B. (2016b). “Chemical compatibility of polymer modified GCLs in coal combustion residual leachates.” Proc., Geoamericas 2016 Conf., International Geosynthetics Society, Jupiter, Fla.
Geosynthetic Institute (2016). “GRI-GCL3: Standard specification for test methods, required properties, and testing frequencies of geosynthetic clay liners (GCLs),” Rev. 4, March 28.
 TEXTILES.ORG
TEXTILES.ORG